Six Factors that weaken chlorine in swimming pools
Slow Chlorine = Weak Chlorine
Sanitization and disinfection are the two primary responsibilities of chlorine. Chlorine’s secondary responsibility–or, more accurately, it’s secondary obligation–is oxidation. In terms of residual sanitizer out in the pool water, chlorine is the first line of defense against common recreational water diseases like pseudomonas aeruginosa; bacteria like e. coli; other germs like staphylococcus aureus and giardia; and living organisms like algae.
So its speed is critical.
If chlorine is slowed down, its kill times, referred to in biochemistry as contact time (CT) are proportionally increased. And that’s not a good thing when it comes to keeping water safe. We want chlorine to kill germs and viruses fast and efficiently. Slow chlorine is weak chlorine, and there are several factors discussed in this article that drag chlorine down.
To help protect people against those nasty waterborne pathogens, there are secondary disinfection systems like UV, but they are a point-of-contact system. In other words, secondary disinfection systems can only affect what they touch, and they only touch what passes through them. Chlorine, on the other hand, is flowing with the water throughout the entire pool and system…it’s everywhere. So let’s get into what slows it down.
Factors that slow chlorine
1. Metals
When chlorine is introduced to the water, it reacts first with metals like iron and manganese. Chlorine oxidizes these metals first and foremost, which in turn knocks out available chlorine early on. The reaction looks like this:
Cl2 + Fe † FeCl2
You can see this on the chart below. Notice that chlorine residual does not present itself until point A. What happened prior to that? Why did it not start building residual from the start? The answer is metals between 0 and point A. The chart labels it as “Destruction of chlorine residual by reducing compounds.” For more information on the science of breakpoint chlorination, read this.

2. Non-living organics (bather waste)
Why are we depending on chlorine alone to remove non-living organic waste from the pool? Chlorine is critical to the safety and wellbeing of everyone in the pool because of disinfection…not removing bather waste. But alas, the bather waste (such as sweat, urine, body oils, mucus, lotions, cosmetics, deodorants and hair gels) must be oxidized to get them out. Right?
Wrong. Oxidation is not the most efficient or effective means of removing bather waste from a swimming pool. Try using SimplyPURE enzymes instead. They are strong enough for wastewater; yet safe enough to meet the NSF/ANSI 50 standard. The enzymes flow throughout the entire pool system–alongside chlorine–and devour organic contamination to reduce the burden on chlorine. By using enzymes, chlorine is freed up to sanitize and disinfect…and that’s what we need it for in a pool.
More specifically than non-living organics, however, are nitrogen compounds. Nitrogen compounds combine with chlorine (to create combined chlorine). The breakpoint chlorination process shows this occurring, then the eventual destruction of combined chlorine compounds like monochloramine and dichloramine. Eventually, with enough hypochlorous acid (HOCl) to break it down and convert it to nitrogen trichloride (aka trichloramine), these compounds will off-gas into the air. They then become an air quality problem.
3. Direct sunlight (UV)

Direct sunlight breaks down chlorine. Without a stabilizer (cyanuric acid) in the water, as much as 90% of free available chlorine could be destroyed within just two or three hours.
We conducted fairly diligent research for this article. We found plenty of sources indicating that direct sunlight breaks down chlorine and bromine; a fact that is irrefutable. What we were looking for, however, we have not yet found. Does anyone know if UV sanitation systems for pools also break down chlorine? And if so, how much? Is it similar or even more severe than sunlight? We think it must be less severe than sunlight, but we honestly do not know. It would be great if a UV manufacturer could contact us and let us know.
But back to what we know. Direct sunlight breaks down chlorine in a matter of hours. Obviously, broken down chlorine is ineffective at sanitation, so therefore sunlight makes this list. Fortunately for outdoor pools, several decades ago a wonderful discovery was made, called…
4. Cyanuric Acid (chlorine stabilizer) Overstabilization
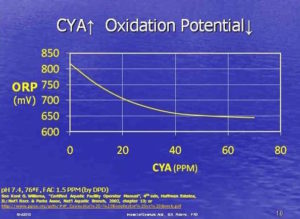
The more stabilizer you have in your pool, the slower the chlorine.
Without cyanuric acid (CYA), chlorine would break down in a matter of hours in direct sunlight. Something about the UV rays breaking apart the HOCl and OCl- itself, but the specific chemistry is not important to this conversation. Just know that without cyanuric acid, also known as chlorine stabilizer, outdoor pools need to be constantly replenishing free chlorine. It’s good to have some stabilizer in an outdoor pool.
The key is moderation. Overstabilization is the problem to avoid, and it’s all about the free chlorine to cyanuric acid ratio (FAC:CYA), which we will discuss in a moment. The US Centers for Disease Control (CDC) released a mandate for regulating the use of cyanuric acid in commercial swimming pools. The new regulation stipulates CYA levels cannot exceed just 15 parts per million! That can be a real problem for pools that use trichlor.
CYA’s dramatic impact on chlorine kill rates
Why limit the use of a stabilizer that protects chlorine from sunlight? Because it also weakens chlorine. Pool industry experts disagree and debate whether or not “chlorine lock up” is a real thing. The notion being that CYA prevents a certain amount of chlorine from being used. The numbers we have heard range between 5-10%, but the best article we have found on the topic of chlorine lock is from Service Industry News. Read the article. It’s worth your time. And we quote:
“Richard Falk derived his ratio in part by recognizing that even if the free chlorine is the same, the concentration of hypochlorous acid (effective chlorine) changes when cyanuric acid is introduced at different levels…
…He did this by recognizing hypochlorous acid, HOCl (the killing form of chlorine) is proportional to the ratio of free chlorine to cyanuric acid:
HOCl ♀ FC/CYA
Beginning with Powell’s best guesses on free chlorine values that are effective for a given cyanuric acid concentration, Falk determined that one should have a minimum free chlorine to cyanuric acid ratio of 7.5 percent to prevent algae in traditionally chlorinated pools.
Falk’s ratio has made doing the math to prevent algae incredibly easy.
FC = 7.5% x CYA
For example, if the measured cyanuric acid in a swimming pool is 30, then a pool operator should maintain a minimum free chlorine level of 2.25 ppm.
2.25 ppm FC = 7.5% x 30 ppm CYA
If the cyanuric acid is at 70 ppm, the free chlorine should be maintained at a minimum of 5.25 ppm.
5.25 ppm FC = 7.5% X 70 ppm CYA
So, assuming Falk’s numbers are correct, the factor of 7.5% is an important one to understand. If your pool has 100ppm cyanuric acid, you basically don’t have free chlorine until you exceed 7.5ppm chlorine. That’s your new baseline. Crazy, right?
5. High pH
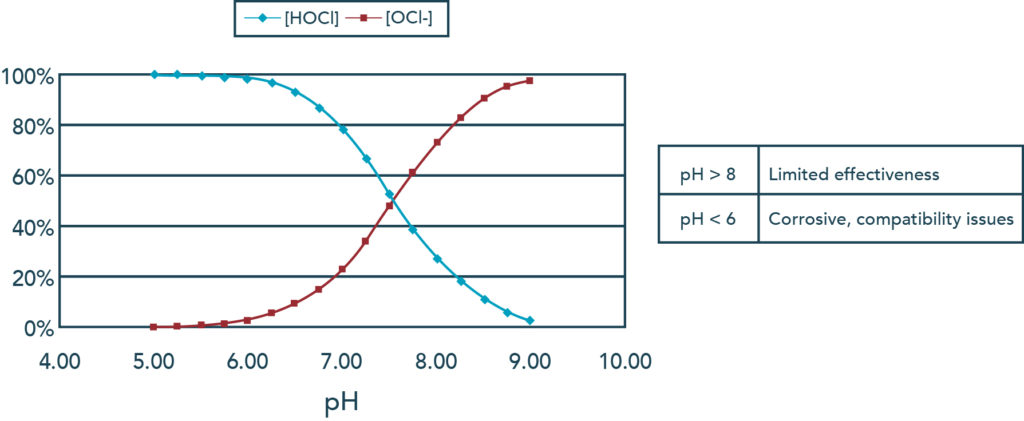
In non-stabilized water (meaning no CYA), The higher the pH, the lower the concentration of HOCl in free chlorine readings. The weaker form of chlorine, hypochlorite ion (OCl-) increases and surpasses HOCl around 7.5 pH.
As you can see from the chart above, pH has a direct impact on the dissociation of H+ from hypochlorous acid in the pool. Hypochlorous acid (HOCl) is the strong, killing form of chlorine We need it in the water! The higher the pH, the less of it there is, as it is replaced by the weaker chlorine, the hypochlorite ion (OCl-). So yes, more alkaline pH can weaken chlorine. Conversely, the lower the pH, the stronger the chlorine, because it has a higher percentage of the strong HOCl.
Let’s be clear about something that sounds somewhat contrarian to industry textbooks and the chart above. The pH of your water dictates HOCl concentrations in pools with no cyanuric acid (CYA). With CYA present, the impact pH has on HOCl concentrations is very little, if not negligible. See the chart below that puts the previous chart in context.
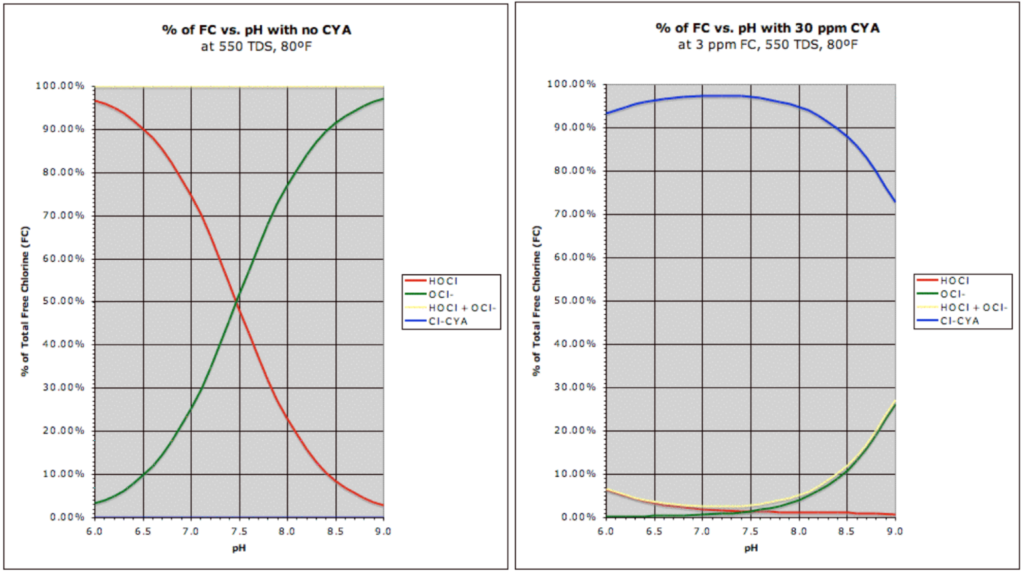
Notice the red line (HOCl concentration) plummets in the presence of CYA. pH now has a negligible impact on HOCl concentration, and chlorine speed/strength is now dictated by CYA. The new blue line at the top of the right graph represents chlorine bound to CYA, also called isocyanurates.
6. Phosphates
 Yes, phosphates indirectly impact chlorine too. Phosphates are an essential micronutrient for organisms to reproduce and grow. In particular, algae. Reproducing algae consumes more and more chlorine in the sanitization battle between the sanitizer and contaminants.
Yes, phosphates indirectly impact chlorine too. Phosphates are an essential micronutrient for organisms to reproduce and grow. In particular, algae. Reproducing algae consumes more and more chlorine in the sanitization battle between the sanitizer and contaminants.
Phosphates are an invisible problem in swimming pools that brings about many consequences. And since chlorine cannot simply oxidize phosphates out of the water, they need to be removed by a dedicated phosphate remover like Blue PRO. Phosphate levels over 500 ppb fuel the reproduction of algae, provided other conditions are met. And while removing phosphates does not kill algae, it can certainly slow its reproduction rate, allowing the sanitizer (chlorine) to stay ahead in the battle, preventing outbreaks.





